Tremor Evaluation Tasks for Steadi-3 Validation Study
Three functional tasks evaluated tremor across movement types:
- Drinking task: lifting and sipping from a cup to assess daily activity tremor.
- Intention tremor task: performing repeated finger-to-nose motions to measure goal-directed movement steadiness.
- Spiral drawing task: completing an Archimedes spiral to assess fine-motor control and tremor impact on handwriting precision.
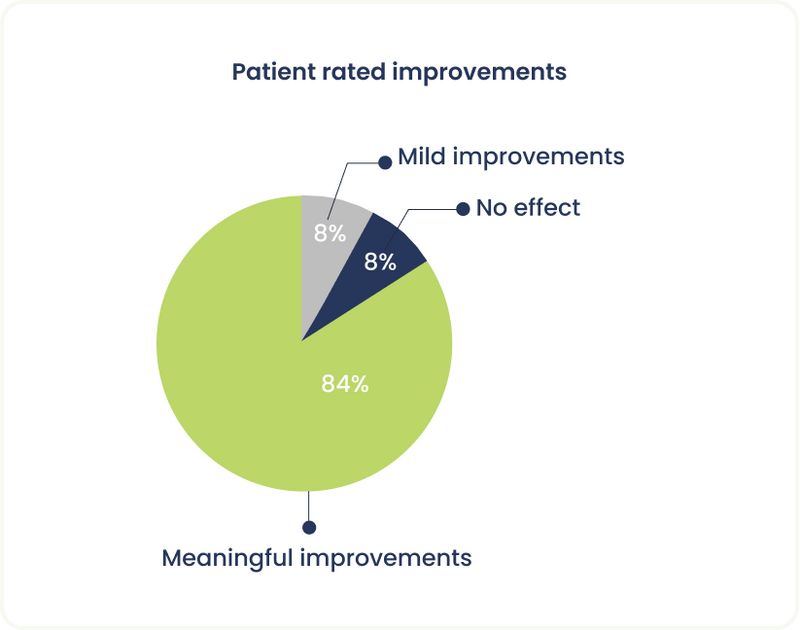

Improved Daily Function After One Week
These functional improvements were particularly noticeable in everyday tasks such as drinking from a cup, eating with utensils, and writing. Supporting these findings, 84% of users reported a significant reduction in tremor severity after just one week of using our assistive device for hand tremors. Importantly, these benefits were achieved without any reported adverse effects, underscoring the device’s safety, ease of use, and real-life impact on daily independence and control.
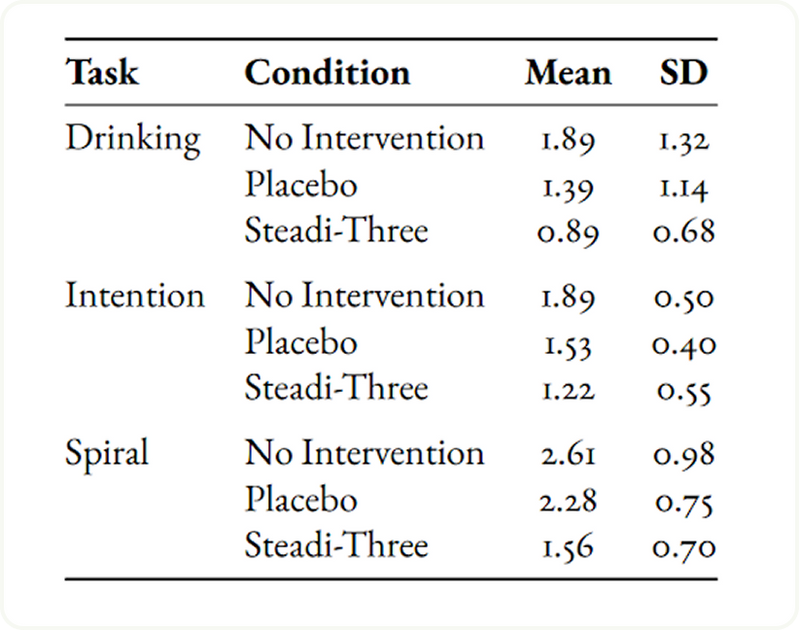

Neurologist-Rated Tremor Severity Across Tasks
In the table, neurologist-rated mean tremor scores (0–4 scale) clearly show that the Steadi-3 device achieved the lowest tremor severity across all activities, with particularly strong results in the drinking task, where mean scores decreased from 1.89 (no intervention) to 0.89 (Steadi-3). Similarly, the intention task improved from 1.89 to 1.22, and the spiral drawing task from 2.61 to 1.56, indicating consistent tremor reduction across both gross and fine motor tasks.
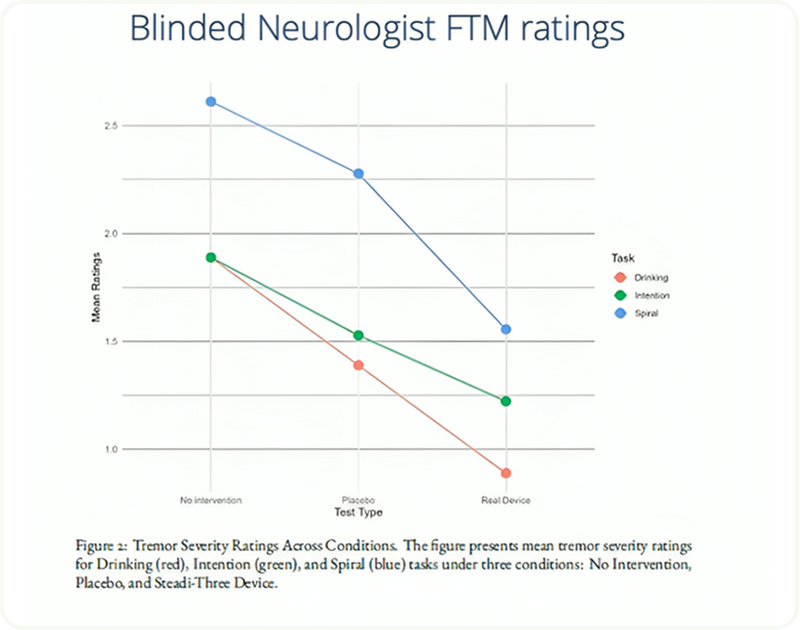

Tremor Severity Reduction
The graph visually reinforces these outcomes, illustrating a steady downward trend in tremor ratings as participants transitioned from baseline to placebo to Steadi-3 conditions. Each colored line—red for drinking, green for intention, and blue for spiral—demonstrates measurable improvement, with the steepest slope observed in the drinking task, reflecting significant enhancement in functional control.
These findings validate Steadi-3’s role in restoring functional independence and facilitating smoother, more controlled motion in everyday life.
Testimonials
Steadi-3 has been proven effective for both conditions. Clinical studies have shown it reduces tremor amplitude in 85% of users, making it a trusted choice for Parkinson's Disease tremor, and Essential Tremor management.
Steadi-3 – Your Anti-Tremor Glove for Daily Independence






Steadiwear Inc
Steadi-3 – Your Anti-Tremor Glove for Daily Independence
Clinically tested glove that reduces hand tremors — battery-free and comfortable for everyday use.
- Stabilizes hand tremors instantly
- Battery-free & lightweight
- 30-day money-back guarantee

30-Day Money Back
Free Shipping
FDA Registered Device
1-Year Warranty
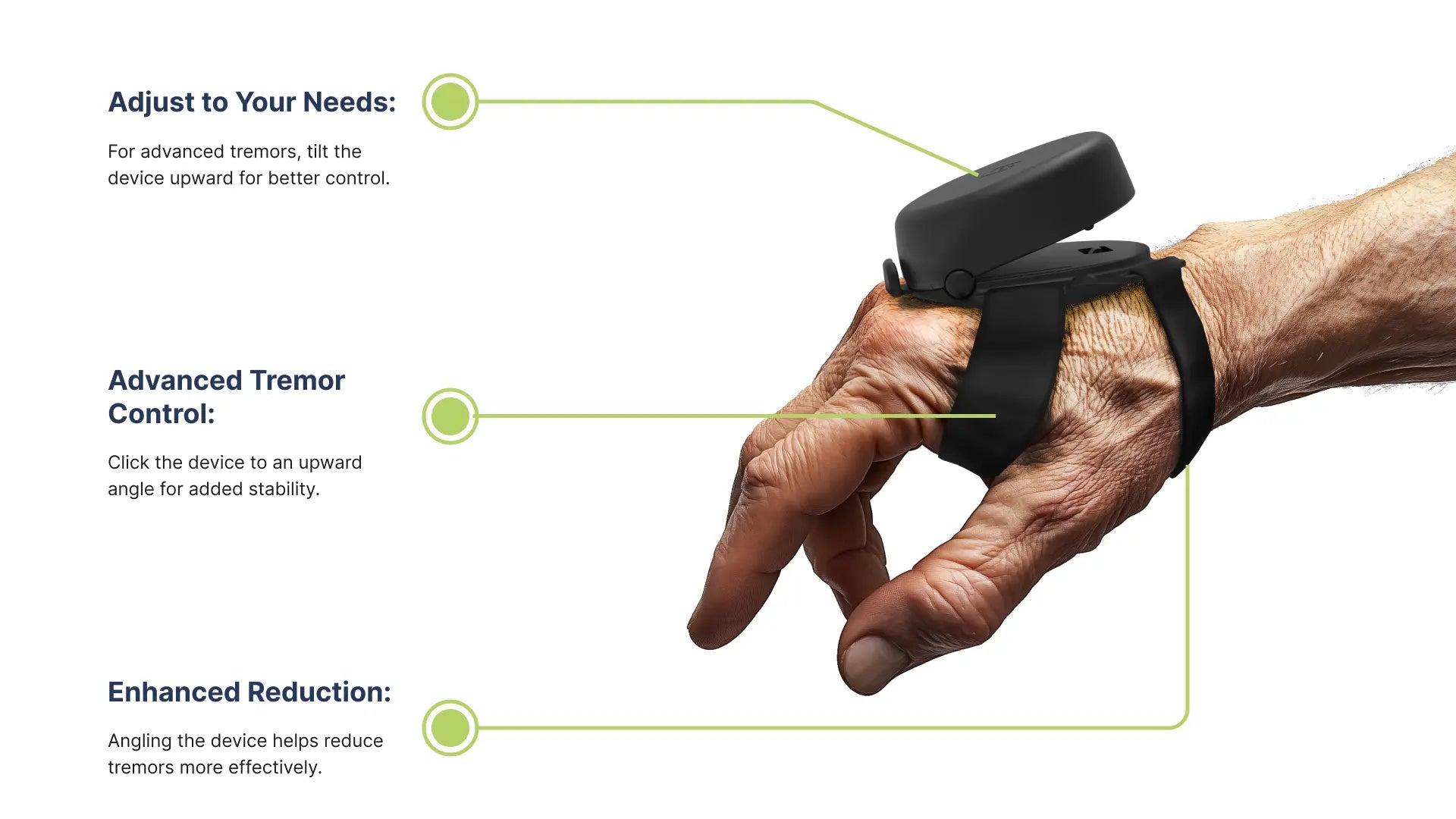
- Universal sizing: Our anti-tremor device is adjustable to meet your hand size. It is available for either hand and ensures an effective experience.
- Compact, Comfortable Fit: With its silicone and Neoprene foam straps, the Steadi-3 tremor-reducing glove is designed to fit securely without compromising comfort, allowing you to wear it throughout the day with ease.
- Automatic Adjustment: The Steadi-3 adapts to your tremor intensity automatically, providing consistent stabilization as your tremor level changes in real time.
- FDA-Registered for Safety: As an FDA-registered Class I medical device, the Steadi-3 tremor-reducing glove is a trusted, safe choice for reducing hand tremors.
- Slide your hand into the glove, ensuring it fits snugly around your palm.
- Tighten the fit by adjusting the velcro until it feels securely in place but still comfortable.
- Once the device is on, it will immediately calibrate itself to your tremor frequency, and starts stabilizing your hands
The Steadi-3 glove offers a simple, battery-free solution designed to provide immediate relief from hand tremors, helping you regain control over daily tasks with ease
In controlled evaluations, 84% of participants showed improved tremor control with Steadi-3 versus no device. 70% showed improvement over placebo, based on blinded neurologist assessments.
Warranty: We back our product with a one-year warranty.
Risk-Free Trial: With our 30-day hassle-free return policy, you can
test the Steadi-3 glove with confidence.
Regain Control: Combat hand tremors effectively with the trusted
Steadi-3 glove from Steadiwear.
Please be aware that all returns are subject to a restocking fee of $83 USD or $117 CAD per unit to cover shipping, returns, customs and fulfillment. This fee will be deducted from your refund in the case of a return request.
If your healthcare provider recommends an assistive device to help manage hand tremors caused by a specific medical condition, you may be able to use your Flexible Spending Account (FSA) or Health Savings Account (HSA) to pay for the Steadi-3 tremor glove.
Learn more here
Frequently Asked Questions
The Steadi-3 evaluation study aimed to assess the device’s effectiveness in reducing upper-limb tremors through controlled testing. Researchers observed participants performing everyday and fine-motor tasks—such as drinking, finger-to-nose movement, and drawing an Archimedes spiral—under three conditions: no intervention, placebo, and active device use. Neurologists rated tremor severity using a modified Fahn-Tolosa-Marin scale, while participants provided feedback on usability and comfort. The study’s goal was to determine whether Steadi-3, as a non-invasive assistive device, could offer significant tremor reduction without medication or surgery, helping individuals with Essential Tremor or related movement disorders regain better control in daily activities.
Tremor severity was evaluated by independent neurologists using a modified Fahn-Tolosa-Marin (FTM) scale, which scores tremor intensity from 0 (no tremor) to 4 (severe tremor). Participants performed three tasks—drinking, intention (finger-to-nose), and spiral drawing—under baseline, placebo, and active device conditions. Videos of each session were reviewed blindly to prevent bias, ensuring that raters could not distinguish between placebo and real device usage. The modified FTM included additional precision for functional tasks like drinking to better reflect real-world usability. This standardized scoring system provided objective, consistent data confirming that the Steadi-3 device significantly reduced tremor across multiple tasks.
The evaluation demonstrated that the Steadi-3 device consistently reduced tremor severity across all tasks compared to both placebo and no intervention. On average, participants’ tremor ratings decreased most notably during the drinking task—from 1.89 at baseline to 0.89 with Steadi-3. The intention task improved from 1.89 to 1.22, and the spiral task from 2.61 to 1.56. Statistical analysis (ANOVA) confirmed significant effects for both task type and intervention condition. Additionally, 70% of participants improved over placebo, 80% over baseline, and 83% self-reported noticeable improvement—indicating strong alignment between clinician evaluations and participant experiences with the device.
Participants reported overwhelmingly positive outcomes. After both short-term and one-week usage, 83% described significant improvement in tremor control and indicated they would continue using the device. They highlighted easier completion of daily tasks—especially drinking without spilling—as a major benefit. None of the participants experienced adverse effects, confirming the device’s comfort and safety during extended wear. Users also appreciated its ease of integration into their daily routines. These results show that Steadi-3 not only reduces tremor severity but is also practical and user-friendly, making it a promising non-invasive option for ongoing tremor management.
Future studies will focus on larger, more diverse participant groups to validate Steadi-3’s effectiveness across different tremor types and severity levels, including Parkinson’s Disease and Essential Tremor. Researchers plan to incorporate objective biomechanical measurements—such as motion sensors and tremor amplitude tracking—to complement neurologist ratings and further quantify mechanical improvements. Development goals include refining ergonomics for better comfort, enhancing adaptability for varied user needs, and testing long-term performance in real-world environments. These next steps aim to strengthen clinical validation and optimize Steadi-3 as a reliable, non-invasive assistive device for individuals living with tremor-related conditions.




